Orbital Diagram For O 2-
Orbital molecular orbitals theory notes wikimedia commons wikipedia upload 7.7: orbital shapes and energies Bonding orbitals molecular electron chemistry orbital antibonding theory function wave bonds bond molecule atomic delocalized covalent values negative diagram delocalization
Ch 1 : Electrons and Orbitals
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron libretexts cl2 second delocalized homonuclear row Chapter 8 section b quantum numbers for electrons Introduction to molecular orbital theory
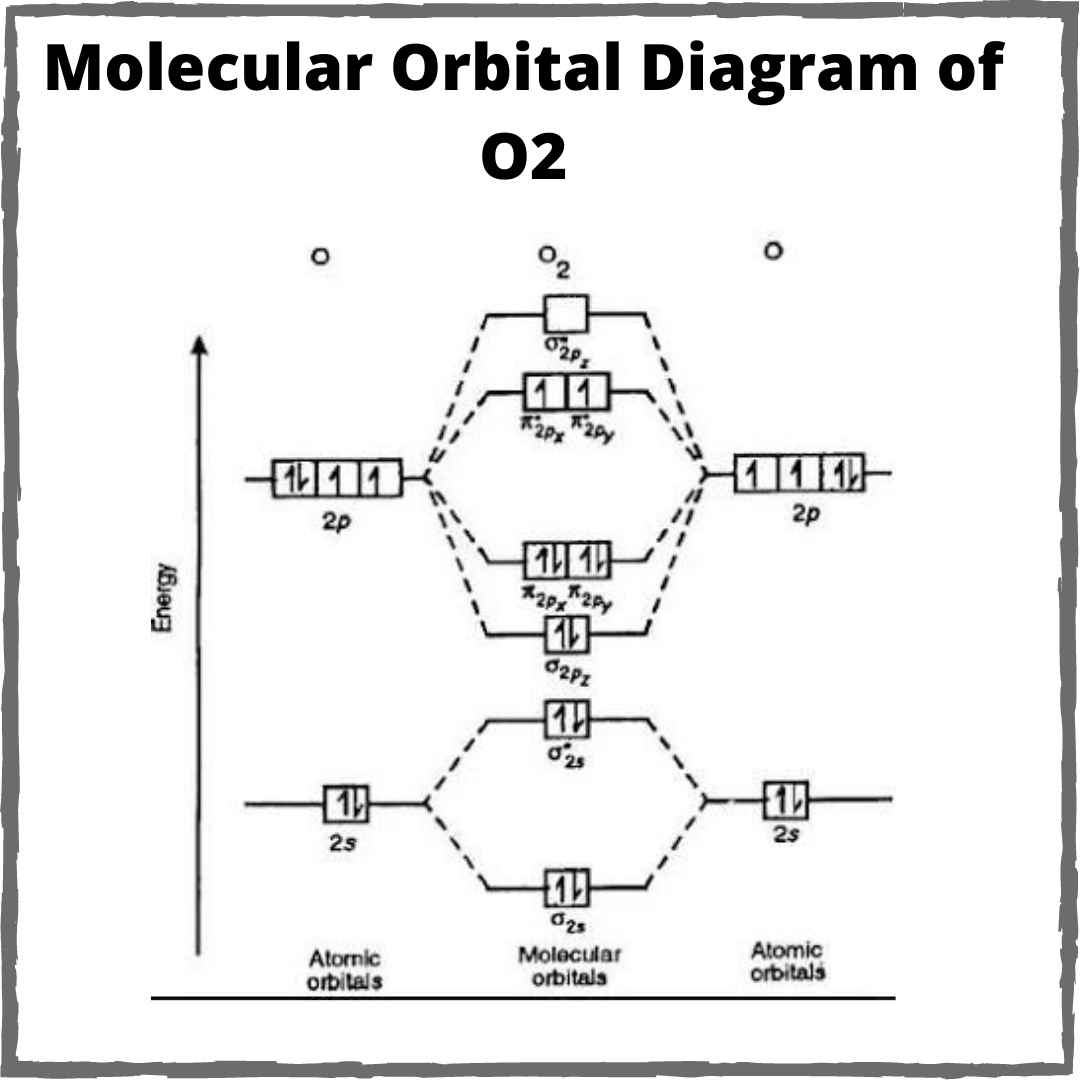
Mo o2 orbital molecular orbitals theory bond paramagnetic diagram oxygen why order configuration atomic energy electrons diagrams unpaired two lone
Orbital orbitals atom theory atomic hydrogen molecular 1s 2s 2p chemistry ic ch ac me chemical bonding same mo plzzzElectron orbitale orbital orbitals atommodelle einiger darstellung atomic quizizz creationwiki dewiki academic Scientia ac labore: molecular orbital theory notes iMolecular chemistry orbitals orbital diagram energy bonding level edu wave chemwiki delocalized h2 theory two bond atomic function molecule libretexts.
Orbitals electron orbital orbitali electrons quantum atomici atomic electronic atoms numeri quantici biopills atom cosa libretexts chimica directional toppr atomoOrbital 2s orbitals quantum electrons 2p familiar coordinate 37+ molecular orbital geometry imageQuantum numbers and electron configurations.

Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formation
Molecular orbital theory38 o2 2- molecular orbital diagram Ch 1 : electrons and orbitalsO2 molecular orbital bond molecule.
Electron probability orbitals quantum shellOrbital orbitals electron shapes single atomic 1s structure figure 2p diagram atom chemistry electronic orbitales electrons 3d diagrams function 4f Why is o2 paramagnetic?9.7: bonding and antibonding orbitals.

9.7: molecular orbitals
.
.

Quantum Numbers and Electron Configurations

Scientia ac Labore: Molecular Orbital Theory Notes I

Chapter 8 Section B Quantum Numbers for Electrons

Ch 1 : Electrons and Orbitals

Orbitale

Why is O2 paramagnetic? | Socratic

9.7: Bonding and Antibonding Orbitals - Chemistry LibreTexts

9.7: Molecular Orbitals - Chemwiki

7.7: Orbital Shapes and Energies - Chemwiki